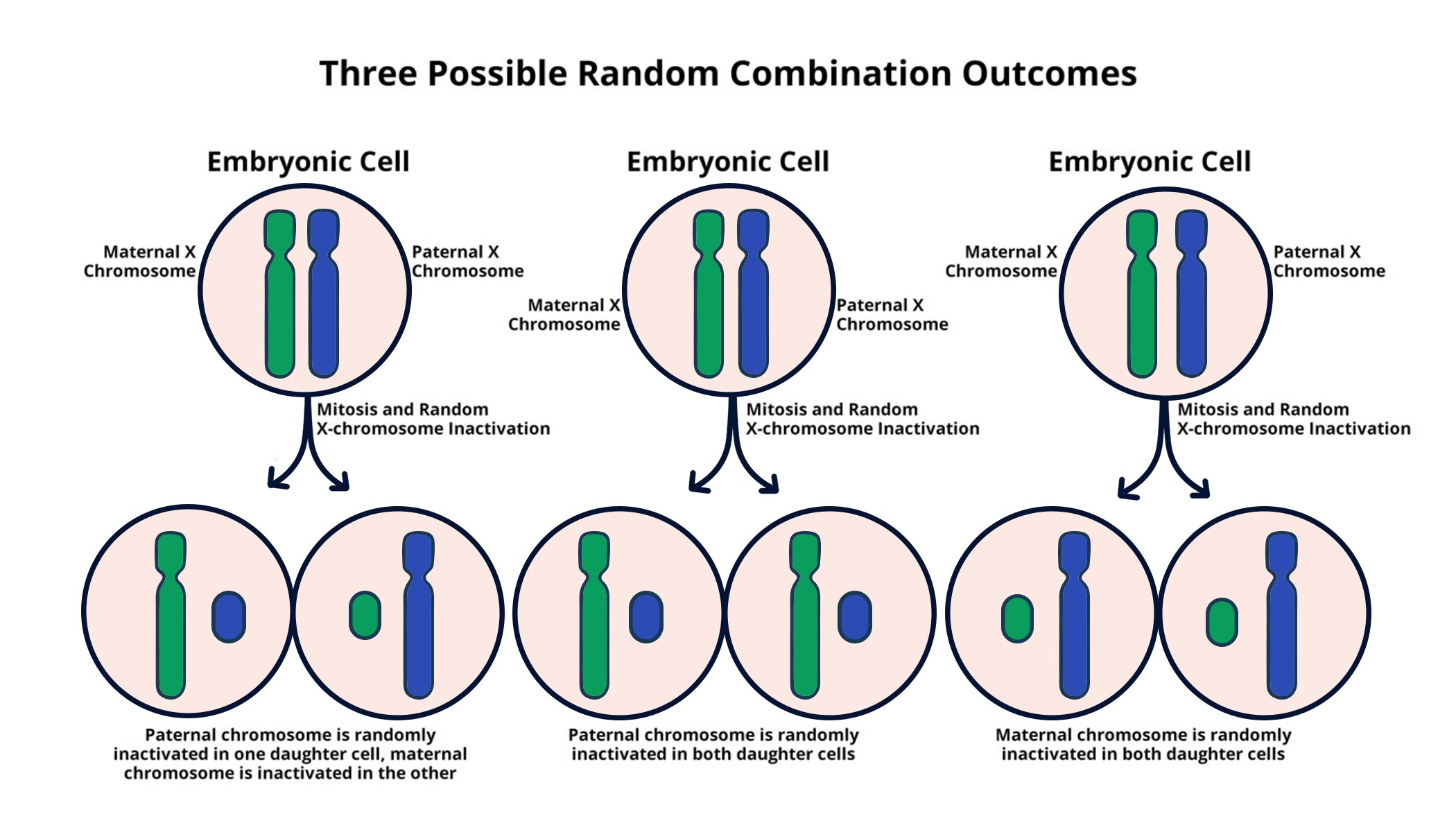
X chromosome inactivation is a critical biological process that helps maintain genetic balance in female mammals, who have two X chromosomes compared to males, who have only one. This complex mechanism ensures that only one copy of the X chromosome remains active, while the other is silenced, preventing an excess of gene dosage that could lead to developmental issues. Researchers, including those in Jeannie T. Lee’s lab, have delved into the intricacies of this chromosomal silencing, revealing the essential role of Xist RNA in orchestrating the inactivation process. Understanding X chromosome inactivation not only sheds light on fundamental genetics but also opens pathways to potential therapies for genetic disorders like Fragile X Syndrome and Rett Syndrome, which are linked to mutations on the X chromosome. By manipulating this process, scientists hope to reactivate mutated genes and provide a better quality of life for affected individuals.
The phenomenon known as X chromosome inactivation, also referred to as lyonization, is a vital adaptive mechanism in the realm of genetics. This process allows female organisms, possessing two X chromosomes, to avoid overexpression of X-linked genes, which could disrupt normal development. It unfolds through the actions of specific molecules, particularly Xist RNA, which play a significant role in chromosomal regulation by inducing the silencing of one X chromosome. This intricate form of chromosomal management has profound implications, particularly for understanding conditions such as Fragile X Syndrome and Rett Syndrome, which arise from mutations in genes located on the X chromosome. By exploring alternative pathways and therapies targeting this mechanism, researchers aim to unlock new treatments for various genetic disorders linked to X-linked mutations.
Understanding X Chromosome Inactivation
X chromosome inactivation is a fascinating genetic process where one of the two X chromosomes in females is silenced, preventing the overexpression of genes solely found on this chromosome. This intricate mechanism is vital for maintaining genetic balance between the sexes, as men possess only one X chromosome. The RNA molecule Xist plays a crucial role in this process by binding to the inactive X chromosome, effectively initiating chromosomal silencing through a unique Tug-of-War interaction with the surrounding chromatin structure, often described as a gelatinous substance. Researchers, including Jeannie Lee, have made significant strides in understanding how this gelatinous casing modifies properties that facilitate X inactivation, marking a breakthrough in cell biology and genetics.
The importance of X chromosome inactivation transcends mere biological curiosity; it holds significant implications for treating genetic disorders. By delving deeper into the mechanics of this phenomenon, scientists have begun exploring ways to reactivate inactivated X chromosomes, which could potentially allow cells to utilize healthy gene copies that were previously silenced. This is especially relevant for conditions like Fragile X Syndrome and Rett Syndrome, where mutations are often confined to one X chromosome. The goal is to engineer methods to reawaken these dormant genes, offering hope for new treatments for individuals grappling with these genetic challenges.
Frequently Asked Questions
What is X chromosome inactivation and why is it important for genetic disorders?
X chromosome inactivation (XCI) is a biological process that occurs in females where one of the two X chromosomes is randomly silenced to ensure equal gene dosage with males, who only have one X chromosome. This mechanism is crucial in the context of genetic disorders such as Fragile X Syndrome and Rett Syndrome, as it can affect the availability of functional genes. Proper XCI helps prevent the overexpression of X-linked genes, while faulty XCI or mutations can lead to significant health issues.
How does Xist RNA contribute to X chromosome inactivation?
Xist RNA plays a pivotal role in X chromosome inactivation by coating the X chromosome and modifying the surrounding chromosomal structure known as ‘Jell-O.’ This modification causes the X chromosome to become inactive, preventing it from expressing genes that could lead to intellectual disabilities and neurodevelopmental issues, such as those seen in Fragile X Syndrome and Rett Syndrome. The dynamics of Xist RNA binding and action are essential for understanding the broader implications of XCI in genetic research.
Can releasing inactivated X chromosomes help treat Fragile X Syndrome and Rett Syndrome?
Yes, by reactivating inactivated X chromosomes, it’s possible to restore the function of healthy genes that may be mutated on the active X chromosome in disorders like Fragile X Syndrome and Rett Syndrome. This approach aims to provide therapeutic benefits by allowing cells to utilize the healthy version of X-linked genes that were previously silenced, potentially curing the associated genetic disorders with minimal side effects.
What are the challenges associated with X chromosome inactivation in genetic research?
Understanding X chromosome inactivation poses significant challenges due to the intricate mechanisms involved, like the interaction between Xist RNA and the chromosomal structure known as ‘Jell-O.’ While researchers have made headway in elucidating these processes, further studies are needed to fully understand how to selectively reactivate genes on the inactivated X chromosome without adversely affecting other essential genes, which is crucial in developing therapies for conditions linked to X-linked genetic disorders.
Are there implications of X chromosome inactivation for male genetic disorders such as Fragile X Syndrome?
Although males do not undergo X chromosome inactivation, they can still be impacted by mutated genes on their single X chromosome, such as those causing Fragile X Syndrome. Understanding the mechanisms of XCI in females helps researchers explore similar patterns of gene silencing in males, potentially leading to new treatment strategies for X-linked disorders regardless of sex.
| Key Points | Details |
|---|---|
| X Chromosome Inactivation | Females have two X chromosomes, but only one is active. This inactivation is essential for balancing gene dosage between genders. |
| Role of Xist RNA | The gene Xist produces an RNA that coats the X chromosome and alters the properties of the surrounding ‘Jell-O’, leading to inactivation. |
| Discovery of Mechanism | Research led by Jeannie T. Lee has unveiled the mechanism behind X chromosome inactivation and its potential therapeutic implications. |
| Potential Treatments | Reactivating the inactivated X chromosome may lead to treatments for disorders like Fragile X Syndrome and Rett Syndrome. |
| Future Clinical Trials | The Lee lab aims to conduct safety studies and clinical trials for these new treatment strategies. |
| Minimal Side Effects | Early indications are that reactivating the healthy genes may not harm the function of other genes on the X chromosome. |
Summary
X chromosome inactivation is a fundamental biological process that ensures dosage compensation between the sexes in humans. It involves the silencing of one of the two X chromosomes in females to prevent the overexpression of genes. Jeannie T. Lee’s groundbreaking work at Harvard Medical School has shed light on the intricate mechanisms behind this process, revealing not only how cells orchestrate X inactivation through the action of Xist RNA and a gelatinous ‘Jell-O’ substance but also the potential for treating X-linked disorders like Fragile X Syndrome and Rett Syndrome. By reactivating inactivated X chromosomes, researchers are paving the way for innovative therapies that could greatly improve the lives of those affected, highlighting the importance of continued research in this promising area.